Graphene-quantum-dot-composited platinum nanotube arrays as a dual efficient electrocatalyst for the oxygen reduction reaction and methanol electro-oxidation†
Abstract
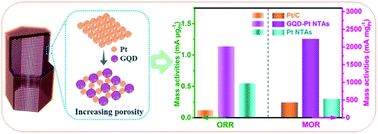
Platinum (Pt) is an excellent electrocatalyst for cathode and anode reactions in direct methanol fuel cells (DMFCs). However, constructing nanostructures with efficient Pt utilization and electrocatalytic activity is a major challenge for the oxygen reduction reaction (ORR) and methanol oxidation reaction (MOR) in an acidic medium. Herein, we describe a facile strategy to prepare high activity graphene quantum dot composited Pt nanotubes arrays (GQD–Pt NTAs) with an available porous surface through an electro-codeposition approach. Benefiting from the difference in size between graphene quantum dots and Pt nanoparticles, the GQD–Pt NTAs possess an adjustable porous structure. Moreover, the increased porosity of the GQD–Pt NTAs improves the effective availability of the Pt surface, significantly enhancing the electrocatalytic activity with a high current density of 1.14 mA μgPt−1 at 0.90 V (vs. RHE) and 2227.08 mA mgPt−1 at 0.85 V (vs. RHE) for the ORR and MOR, respectively. This work suggests that combining Pt with nonmetallic quantum dots is indeed an effective approach to address the unsatisfactory utilization and electroactivity of Pt-based nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...