Activity of layered swedenborgite structured Y0.8Er0.2BaCo3.2Ga0.8O7+δ for oxygen electrode reactions in at intermediate temperature reversible ceramic cells†
Abstract
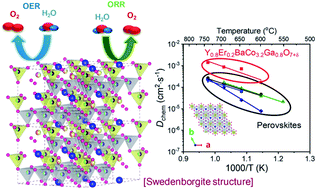
To improve the thermal stability and intrinsically sluggish kinetics of oxygen electrode reactions in solid oxide fuel cells (SOFCs) and reversible protonic ceramic cells (RPCCs) at intermediate temperatures, a novel layered swedenborgite structure Y0.8Er0.2BaCo3.2Ga0.8O7+δ (YEBCG) catalyst is introduced as an alternative to perovskite materials that contain cobalt. The thermal expansion coefficient of YEBCG is 8.41 × 10−6 K−1, which is relatively well matched to the state-of-the-art proton-conducting and oxygen-ion-conducting electrolytes. The chemical bulk diffusion and surface exchange coefficients of YEBCG are 7.12 × 10−4 and 8.01 × 10−3 cm2 s−1, respectively, at 650 °C, which leads to much faster action than with state-of-art perovskite structured materials at intermediate temperatures. The maximum power densities of YEBCG cells are notably high, reaching 0.77 and 0.83 W cm−2 at 650 °C in SOFC and protonic ceramic fuel cell modes, respectively. Under electrolysis, the YEBCG cells achieve outstanding current densities of −0.61 and −4.42 A cm−2 at 500 and 700 °C, respectively, under an applied voltage of 1.4 V. Furthermore, the RPCC with YEBCG present no degradation over an entire 1000 h in fuel cell and electrolysis cell modes. These results demonstrate the excellent properties, including good durability, of the YEBCG air electrode when used in high performance SOFCs and RPCCs.


 Please wait while we load your content...
Please wait while we load your content...