Electrochemical synthesis of ammonia from nitrogen catalyzed by CoMoO4 nanorods under ambient conditions†
Abstract
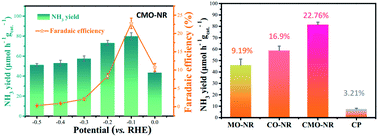
Exploring efficient and durable noble-metal-free electrocatalysts is a promising direction for the electrocatalytic N2 reduction reaction (e-NRR) under ambient conditions. Herein, CoMoO4 nanorods (CMO-NR) were for the first time synthesized for electrochemical N2 fixation to produce NH3 through an easy operating hydrothermal and low-temperature oxidization strategy. Systematic experiments revealed that the bimetallic CMO-NR electrocatalyst exhibited synergistically improved e-NRR performance with high faradaic efficiency (22.76%) and NH3 yield (79.87 μmol h−1 gcat.−1), and is superior to the monometallic counterparts (CoO and MoO3 nanorods). Moreover, electrochemical stability was also achieved over CMO-NR during a long-time e-NRR test. This work can serve as a reference for establishing methods of designing bimetallic oxides towards catalyzing the e-NRR.


 Please wait while we load your content...
Please wait while we load your content...