Two-dimensional materials as a stabilized interphase for the solid-state electrolyte Li10GeP2S12 in lithium metal batteries†
Abstract
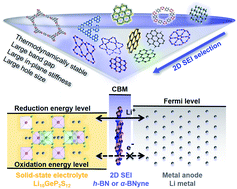
Superionic conductor Li10GeP2S12 has a high lithium-ion conductivity of 12 mS cm−1 at room temperature, but its poor chemical stability against lithium metal anodes restricts its practical use. Introducing suitable two-dimensional (2D) sheets between Li10GeP2S12 and a lithium metal anode as a solid-electrolyte interphase (SEI) is expected to solve the interfacial problem without sacrificing energy density. A set of systematic selection schemes for 2D SEIs involving thermodynamic, electronic, and mechanical properties is proposed. h-BN and α-BNyne are finally selected, and first-principles calculations are carefully performed. Compared with the case where Li10GeP2S12 is directly exposed to a lithium metal anode, the two interfaces added by the ultrathin h-BN or α-BNyne monolayer as a SEI become chemically stabilized, with the electrochemical windows expanded by 2.08–2.41 eV. Significantly higher and wider barriers for electrons form while maintaining acceptable Li-ion diffusion barriers at the interfaces. The simple theoretical 2D SEI screening scheme exhibited here can be extended to other solid-state battery systems.


 Please wait while we load your content...
Please wait while we load your content...