Interaction of proteins with phase boundaries in aqueous two-phase systems under electric fields
Abstract
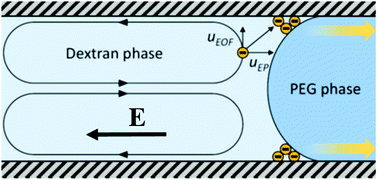
The electric-field driven transport of proteins across the liquid–liquid interface in an aqueous two-phase system (ATPS) is studied in a microfluidic device using fluorescence microscopy. An ATPS containing polyethylene glycol (PEG) and dextran is employed, and bovine serum albumin (BSA) and bovine γ-globulins (BγG) are considered as model proteins. It is shown that both proteins, initially in the dextran-rich phase, accumulate at the liquid–liquid interface, preferably close to the three-phase contact line between the two liquid phases and the microchannel wall. It is in these regions where the proteins penetrate into the PEG-rich phase. The transport resistance of the liquid–liquid interface is higher for BγG than for BSA, such that a much larger molar flux of BSA into the PEG phase is observed. This opens up the opportunity of separating different protein species by utilizing differences in the transport resistance at the interface. A mathematical model is developed, accounting for adsorption and desorption processes at the liquid–liquid interface. The underlying theoretical concept is that of an electrostatic potential minimum formed by superposing the applied electric field and the field due to the Donnan potential at the interface. A fit of the model parameters to the experimental data results in good agreement between theory and experiments, thereby corroborating the underlying picture.

 Please wait while we load your content...
Please wait while we load your content...