Photocatalyst- and additive-free decarboxylative alkylation of N-aryl tetrahydroisoquinolines induced by visible light†
Abstract
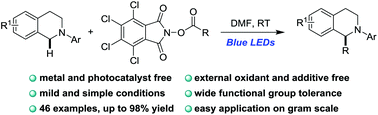
A visible light induced decarboxylative alkylation of N-aryl tetrahydroisoquinolines has been developed using tetrachloro-N-hydroxyphthalimide esters as alkylation agents. This redox-neutral reaction can proceed without the assistance of photocatalysts, transition metals and external additives by irradiation with blue LEDs. Preliminary mechanistic studies indicated that this transformation probably proceeded through an electron donor–acceptor complex involved radical process.


 Please wait while we load your content...
Please wait while we load your content...