Design and organocatalytic synthesis of spirooxindole–cyclopentene–isoxazole hybrids as novel MDM2–p53 inhibitors†
Abstract
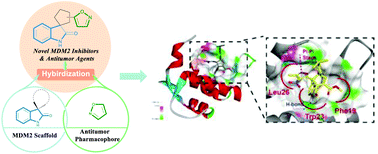
A collection of spirooxindole–cyclopentene–isoxazole hybrids bearing multiple chiral centers and various groups as potential MDM2–p53 inhibitors were designed and synthesized via a rarely reported organocatalytic 1,6-cycloaddition with exclusive α-regioselectivity. In vitro MDM2 binding and cytotoxicity assays showed that compound 4z was the most potent MDM2 inhibitor, while a mechanistic study indicated that oxidative damage and apoptosis in p53-wild colorectal cancer cells were induced. Molecular docking suggested that the H-bonds with Leu54 and pi stacking with His96 were crucial interactions between 4z and the substrate binding site of MDM2. This article demonstrates an efficient protocol to design and synthesize potent biologically active compounds, providing a basis for developing novel MDM2 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...