Synthesis and redox reactions of binuclear zinc(ii)–thiolate complexes with elemental sulfur†
Abstract
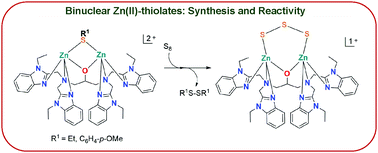
The synthesis and characterization of a series of new binuclear Zn(II) complexes bearing (i) terminal H2O/OH, (ii) bridging thiolates, (iii) bridging thiocarboxylates, (iv) bridging carboxylates, and (v) bridging carboxylate bearing free thiol groups are presented. Unlike the previously reported Co(II) and Fe(II) mediated desulfurization of thiolates to yield binuclear Co(II)/Fe(II)-hydrosulfide complexes by us (Jana et al., Inorg. Chem., 2018, 57, 617; Pal et al., Dalton Trans., 2019, 48, 5903), Zn(II) was found to be incapable of mediating the C–S bond cleavage of thiolates and thus allowed the isolation of binuclear Zn(II)–thiolate complexes. These binuclear Zn(II)–thiolate complexes were further examined for chemical oxidation of the coordinated thiolates and insertion of neutral sulfur into the Zn(II)–thiolate bonds. Interestingly, the latter reactivity study demonstrated the formation of a new binuclear Zn(II) complex featuring a bridging S32− unit which was generated via a redox reaction involving the bridging thiolates and the externally added elemental sulfur.


 Please wait while we load your content...
Please wait while we load your content...