Direct conversion of secondary propargyl alcohols into 1,3-di-arylpropanone via DBU promoted redox isomerization and palladium assisted chemoselective hydrogenation in a single pot operation†‡
Abstract
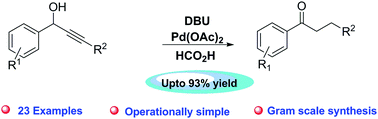
Palladium(II)acetate is found to be an efficient catalyst for the single-step conversion of secondary propargyl alcohols to 1,3-diarylpropanone derivatives under mild basic conditions. The reaction is believed to proceed via redox isomerisation of secondary propargyl alcohols followed by chemoselective reduction of an enone double bond with formic acid as an adequate hydrogen donor. A large number of 1,3-diarylpropanone derivatives may readily be prepared from a milligram to a multigram scale.


 Please wait while we load your content...
Please wait while we load your content...