Bio-based photo-reversible self-healing polymer designed from lignin†
Abstract
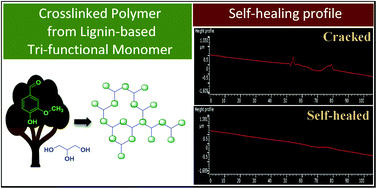
Lignocellulose-derived p-hydroxycinnamic acids possess the photo-responsive reversible α,β-unsaturated ester moiety, a reactive structural feature that functions as the basis of the photo-reversible [2 + 2] cycloaddition reaction. To explore the potential of these naturally occurring compounds in the field of self-healing materials, novel bio-based photo-crosslinkable lignin- and glycerol-derived polyfunctional monomers having cinnamate groups were produced using a sustainable process from vanillin and syringaldehyde, two compounds readily obtained from the oxidation of lignin. Through a Structure–Activity Relationship (SAR) study, the structural design of these bio-based monomers was optimized with regards to the crosslinking/decrosslinking extent and the self-healing capacity of the corresponding polymer material. The lignin- and glycerol-derived tri-functional monomer with 4-O-propyl-ferulate moieties has facilitated the design of a new family of bio-based, environment-friendly and reversible self-healing materials for widespread applications. Computational density-functional theory (DFT) and time-dependent DFT calculations were further used for the verification of the SAR study in terms of dimerization energy of the synthesized monomers.


 Please wait while we load your content...
Please wait while we load your content...