Regeneration of LiFePO4 from spent lithium-ion batteries via a facile process featuring acid leaching and hydrothermal synthesis†
Abstract
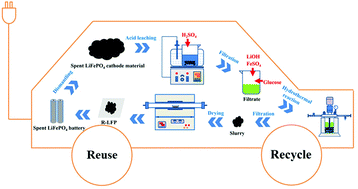
Due to them being widely used batteries, a boom in the disposal of LiFePO4 (LFP) batteries is coming. Both pyrometallurgical repair and hydrometallurgical processes have been applied in the recycling of spent LFP batteries. The former has limited application due to its poor adaptability for spent LIBs with different chemistries or inconsistent degrees of damage, while the latter has poor economic returns due to the low added value of its products, ferric phosphate and lithium carbonate. In this study, a facile process for directly regenerating LiFePO4 from spent LFP cathode material involving acid leaching and hydrothermal synthesis is proposed. The results indicate that the acid leaching process of the spent LFP cathode material depends on the surface chemical reaction, and that 96.67% lithium and 93.25% iron leaching efficiency can be simultaneously achieved by control of the thermodynamic conditions. Additionally, through component control and a one-step hydrothermal process, a flake-like LFP cathode material was successfully regenerated directly from the leaching solution. The regenerated LFP cathode material shows good electrochemical performance, and its discharge capacity is 136 mA h g−1 at 0.1 C. After 300 cycles at 1 C, its capacity retention ratio is as high as 98.6%. Moreover, an economic evaluation indicates that the process is profitable. Therefore, the proposed approach in this study could help to avoid the complex element separation process and achieve the facile recycling of LFP cathode material, thus providing a novel and efficient method for the clean and economical recycling of spent LFP cathodes.


 Please wait while we load your content...
Please wait while we load your content...