Synthesis and characterization of heteroleptic rare earth double-decker complexes involving tetradiazepinoporphyrazine and phthalocyanine macrocycles†‡
Abstract
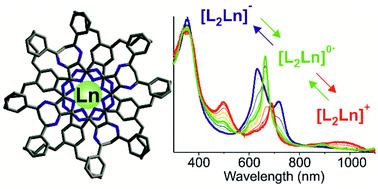
Reaction of (2,3,9,10,16,17,23,24-octabutylphthalocyaninato)lanthanide(III) acetylacetonates (BuPcLn(acac), 1a–c, Ln = Lu (a), Eu (b), La (c)) with a tetrakis(5,7-bis(4-tert-butylphenyl)-6H-1,4-diazepino)[2,3-b,g,l,q]porphyrazine ligand (tBuPhDzPzH2, 2) produced sandwich compounds (tBuPhDzPz)Ln(BuPc) (3a–c), which represent the first heteroleptic double-deckers incorporating both Pc and DzPz decks. A combination of high-resolution mass spectrometry, UV–Vis/NIR, MCD, and 1H NMR spectroscopy, and square-wave voltammetry provided unambiguous characterization of target complexes 3 indicating that their spectral and electrochemical properties are generally intermediate with respect to their homoleptic relatives. Based on the data of solution-state 1H–1H NMR (COSY, NOESY) correlation spectroscopy supported by DFT calculations, a dimerization tendency of compounds 3 proportional to the Ln(III) ion size was found. The spectroelectrochemical study of 3 and the corresponding homoleptic double-deckers revealed a pronounced tendency to aggregation of the one-electron oxidized forms of DzPz-containing double-decker complexes compared to homoleptic Pc2Ln compounds.


 Please wait while we load your content...
Please wait while we load your content...