Enhanced activity and alkali metal resistance in vanadium SCR catalyst via co-modification with Mo and Sb†
Abstract
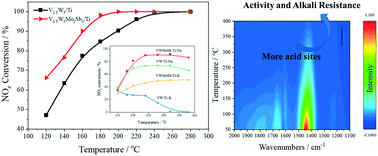
A series of V0.04W0.03MoxSby/TiO2 catalysts was prepared by impregnating various contents of Mo and Sb. The optimum V0.04W0.03Mo0.015Sb0.018/TiO2 catalyst exhibited enhanced SCR activity in a wide temperature range of 160–320 °C, together with excellent SO2 and H2O resistance. The addition of an appropriate amount of Mo and Sb could strengthen the alkali metal resistance of the V0.04W0.03/TiO2 catalyst. The Mo and Sb co-modified catalyst exhibited a large specific surface area. The XPS and EPR results confirmed the presence of abundant V4+ and Oβ on the modified catalyst. The TPR and TPD experiments revealed that the enhanced low-temperature redox ability and improved surface acidity played a key role in the superior catalytic activity and alkali metal resistance over the V0.04W0.03Mo0.015Sb0.018/TiO2 catalyst. The in situ DRIFTS results revealed that the reaction mechanism followed the E–R mechanism over the V0.04W0.03Mo0.015Sb0.018/TiO2 catalyst at 180 °C. Additionally, the inhibitory effect of K was mainly attributed to the destruction of the redox ability and surface acidity of the catalyst.

 Please wait while we load your content...
Please wait while we load your content...