Elucidation of the mechanism and structure–reactivity relationship in zeolite catalyzed alkylation of benzene with propylene†
Abstract
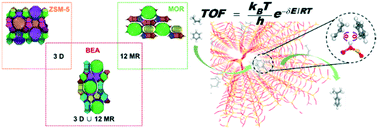
Structure–reactivity relationship is an important concept in catalysis. Herein, the alkylation of benzene with propylene is selected as a model reaction to illustrate the effect of zeolite frameworks on the reactivity. Employing H-BEA, H-ZSM-5 and H-MOR as zeolite catalysts, both stepwise and concerted mechanisms are systematically investigated. The concerted pathway is kinetically more favorable over the three zeolites while the increased contribution of the stepwise pathway is observed with temperature rising. However, these three zeolites exhibit different activities under the dominated concerted mechanism, which are mainly attributed to the different co-adsorption configurations of benzene and propylene around the acidic site. For H-BEA and H-ZSM-5, propylene is firstly protonated and then followed by the electrophilic attack on benzene nearby. As for the specific co-adsorption configuration in H-MOR, benzene and propylene need to move or rotate largely to form the protonated cumene, which results in a larger enthalpy barrier. Temperature has a more significant effect on the activity than pressure and it's mainly the entropy part that dominates the variation of reaction rates at different temperatures. For the domination of the concerted mechanism, our study provides a possibility to further improve reaction activity by subtly tailoring the zeolite structure. The distribution of multi-propylbenzenes (MPBs) around the acidic site reveals that H-ZSM-5 with a smaller channel size will be deactivated more easily than H-BEA. Therefore, in consideration of the activity and stability, H-BEA performs better than H-ZSM-5 and H-MOR, indicating that a zeolite with large tridimensional channels is more suitable for the alkylation of benzene with propylene. Furthermore, the transferred charge between the hydrocarbon and zeolite mainly via the acidic proton is found in these two mechanisms and the hydrocarbon acts as a charge donor firstly and then becomes an acceptor as the reaction proceeds. It's revealed that the stepwise pathway can be regarded as a coupling of two different pathways with or without benzene involved.


 Please wait while we load your content...
Please wait while we load your content...