Electronic and thermodynamic properties of native point defects in V2O5: a first-principles study†
Abstract
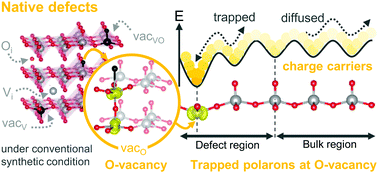
The formation of native point defects in semiconductors and their behaviors play a crucial role in material properties. Although the native defects of V2O5 include vacancies, self-interstitials, and antisites, only oxygen vacancies have been extensively explored. In this work, we carried out first-principles calculations to systematically study the properties of possible native defects in V2O5. The electronic structure and the formation energy of each defect were calculated using the DFT+U method. Defect concentrations were estimated using a statistical model with a constraint of charge neutrality. We found that the vanadyl vacancy is a shallow acceptor that could supply holes to the system. However, the intrinsic p-type doping in V2O5 hardly occurred because the vanadyl vacancy could be readily compensated by the more stable donor, i.e., the oxygen vacancy and oxygen interstitial, instead of holes. The oxygen vacancy is the most dominant defect under oxygen-deficient conditions. However, under extreme O-rich conditions, a deep donor of oxygen interstitial becomes the major defect species. The dominant oxygen vacancy under synthesized conditions plays an important role in determining the electronic conductivity of V2O5. It induces the formation of compensating electron polarons. The polarons are trapped at V centers close to the vacancy site with the effective escaping barriers of around 0.6 eV. Such barriers are higher than that of the isolated polaron hopping (0.2 eV). The estimated polaron mobilities obtained from kinetic Monte Carlo simulations confirmed that oxygen vacancies act as polaron-trapping sites, which diminishes the polaron mobility by 4 orders of magnitude. Nevertheless, when the sample is synthesized at elevated temperatures, a number of thermally activated polarons in samples are quite high due to the high concentrations of oxygen vacancies. These polarons can contribute as charge carriers of intrinsic n-type semiconducting V2O5.


 Please wait while we load your content...
Please wait while we load your content...