Transformation from triple helicene to double helicene embedding adjacent stereogenic carbon atoms and axial stereogenicity†
Abstract
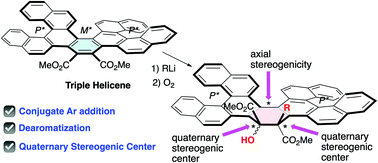
Transformation of triple helicene (TH) to double helicene (DH) with adjacent stereogenic carbon atoms and axial stereogenicity was achieved by the unexpected conjugate addition to the central aromatic ring of TH-1. We also studied the boundary at which different reactivities to addition reactions occur in some helicenes with different π-extension.


 Please wait while we load your content...
Please wait while we load your content...