Biomimetic polydopamine-laced hydroxyapatite collagen material orients osteoclast behavior to an anti-resorptive pattern without compromising osteoclasts’ coupling to osteoblasts†
Abstract
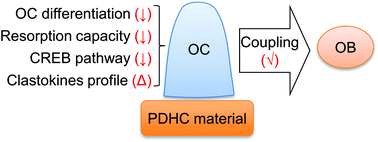
Polydopamine-assisted modification for bone substitute materials has recently shown great application potential in bone tissue engineering due to its excellent biocompatibility and adhesive properties. A scaffold material's impact on osteoclasts is equally as important as its impact on osteoblasts when considering tissue engineering for bone defect repair, as healthy bone regeneration requires an orchestrated coupling between osteoclasts and osteoblasts. How polydopamine-functionalized bone substitute materials modulate the activity of osteoblast lineage cells has been extensively investigated, but much less is known about their impact on osteoclasts. Moreover, most of the polydopamine-functionalized materials would need to additionally load a biomolecule to exert the modulation on osteoclast activity. Herein, we demonstrated that our biomimetic polydopamine-laced hydroxyapatite collagen (PDHC) scaffold material, which does not need to load additional bioactive agent, is sufficiently able to modulate osteoclast activity in vitro. First, PDHC showed an anti-resorptive potential, characterized by decreased osteoclast differentiation and resorption capacity and changes in osteoclasts’ transcriptome profile. Next, cAMP response element-binding protein (CREB) activity was found to mediate PDHC's anti-osteoclastogenic effect. Finally, although PDHC altered clastokines expression pattern of osteoclasts, as revealed by transcriptomic and secretomic analysis, osteoclasts’ coupling to osteoblasts was not compromised by PDHC. Collectively, this study demonstrated the PDHC material orients osteoclast behavior to an anti-resorptive pattern without compromising osteoclasts’ coupling to osteoblasts. Such a feature is favorable for the net increase of bone mass, which endows the PDHC material with great application potential in preclinical/clinical bone defect repair.


 Please wait while we load your content...
Please wait while we load your content...