Synergy of surface fluorine and oxygen vacancy of TiO2 nanosheets for O2 activation in selective photocatalytic organic transformations†
Abstract
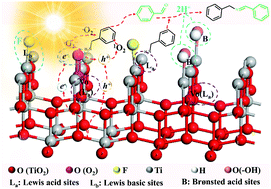
Surface fluorinated TiO2 has received extensive attention in recent years due to the improved photocatalytic reactivity compared with that of pure TiO2. However, the synergy of surface fluorine (FS) and oxygen vacancy (VO) is not clear and needs further study. Here we systematically studied the effect of FS, VO and acidic sites of TiO2 nanosheets on the selective photocatalytic oxidation of benzylamine to imine. The photocatalytic activity first increased and then decreased as the content of FS decreased and VO increased. The photocatalytic activity reached a summit with the imine yield of 92.1% over TiO2-B-6 h, which is mainly due to the synergy between FS and adjacent VO, forming the frustrated Lewis pair that boosts the activation of O2. The varied contents of FS and VO not only cause an evident change in the amount of acidic sites, but also affect the band structure and separation efficiency of charge carriers. The experimental and calculation results demonstrate that the coexistence of FS and VO boosted the separation efficiency, density and mobility of photogenerated charge carriers, resulting in the improved photocatalytic activity. The improved photocatalytic activity can be attributed to the combined effects of FS, VO and acidic sites. This work provides a strategy for the explicit regulation of surface species on TiO2 nanosheets for improved photocatalytic activity.


 Please wait while we load your content...
Please wait while we load your content...