Regulating molecular orientations of dipyran-based nonfullerene acceptors through side-chain engineering at the π-bridge†
Abstract
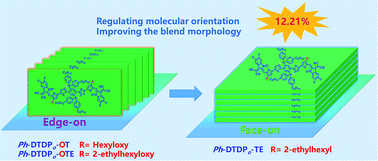
Molecular orientation is one of the key parameters that would greatly influence the charge transportation in sandwich-type organic photovoltaic devices. Herein, two new A–π–D–π–A type acceptor molecules Ph-DTDPo-OTE and Ph-DTDPo-TE are designed and synthesized based on a 2H-pyran derived ladder-type dipyran core (DTDPo). A branched 2-ethylhexyloxy chain is utilized to replace the linear hexyloxy one so as to improve the solubility and blend morphology. It's interesting that the dominant edge-on orientation for Ph-DTDPo-OTE could be converted to the favorite face-on packing when the alkyloxy chains are replaced by alkyl ones. The desired molecular orientations of both Ph-DTDPo-TE and PBDB-T could guarantee the efficient charge transportation. In addition, good miscibility and interpenetrated fibrils have been observed in the blend of Ph-DTDPo-TE:PBDB-T by both surficial and in-depth morphology characterization experiments. Finally, a high PCE of 12.21% (Voc = 0.82 V, Jsc = 20.83 mA cm−2 and FF = 71.14%) is achieved for Ph-DTDPo-TE, which is one of the highest values for binary OSCs based on a pyran comprising acceptor. Our research demonstrates that appropriate side chain engineering at the π-bridge is an effective strategy to regulate molecular orientations and the morphology of blend films.


 Please wait while we load your content...
Please wait while we load your content...