Building an artificial solid electrolyte interphase with high-uniformity and fast ion diffusion for ultralong-life sodium metal anodes†
Abstract
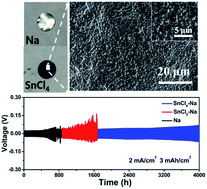
Na metal is regarded as a promising anode for Na batteries owing to its high specific capacity and natural abundance. However, rapid dendrite growth and low reversibility hinder its practical applications. Building an artificial solid electrolyte interphase (SEI) is an effective strategy to stabilize a Na metal anode, yet it still does not achieve high current density and cycling capacity. Here, two artificial SEI layers were constructed through analogous reactions between Na metal and pure SnCl4 liquid (SnCl4–Na electrode) or diglyme solvent with an SnCl2 additive (SnCl2–Na electrode) to investigate the key parameters for an excellent artificial SEI. Both simulations and experiments demonstrate that the high-uniformity and high Na+ diffusion properties of the SnCl4-derived protective layer are significant for stabilizing Na metal anodes under rigorous test conditions. Therefore, the SnCl4–Na electrode protected by a homogeneous and high Na+ diffusion Na–Sn alloy and NaCl achieves an ultralong cycle life of 4000 h and small voltage hysteresis (∼100 mV) with a cycling capacity of 3 mA h cm−2 at 2 mA cm−2, which is much better than that of the SnCl2–Na electrode. Moreover, even with a cycling capacity of 5 mA h cm−2 at 5 mA cm−2, the SnCl4–Na electrode can stably run for ∼1500 h. Benefiting from the durable SEI layer, a stable capacity of ∼350 mA h g−1 is observed on a SnCl4–Na|FeS2 full cell for 380 cycles.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...