Nano vs. bulk rutile TiO2:N,F in Z-scheme overall water splitting under visible light†
Abstract
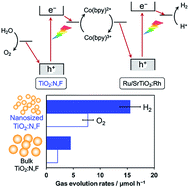
Nanosized semiconductors are potentially efficient photocatalysts because of the short migration distance of excited charge carriers to their surface and their high surface area, both of which positively influence catalytic activity. Although the observation that “nano beats bulk” has been reported for several oxide photocatalysts, very few studies have demonstrated a similar tendency in oxynitrides (including nitrogen-doped oxides) for overall water splitting. Here, we report a rare example of nanosized nitrogen/fluorine-codoped rutile TiO2 (nano-TiO2:N,F) as an O2-evolution photocatalyst in a visible-light-driven Z-scheme water-splitting system in combination with Ru/SrTiO3:Rh and in the presence of redox mediator [Co(bpy)3]3+/2+ (bpy = 2,2′-bipyridine). Utilization of nano-TiO2:N,F improved the activity of the Z-scheme system by a factor of 3–4 compared with bulk-sized TiO2:N,F (bulk-TiO2:N,F). The performance of the optimized nano-TiO2:N,F-based system was twice as high as that of the system constructed with previously reported tantalum/nitrogen-codoped rutile TiO2 under a cocatalyst-free condition and was comparable to that of a system based on a benchmark BiVO4 photocatalyst. Physicochemical analyses revealed that the high surface area and high density of reactive electrons in the nano-TiO2:N,F both contributed to its high photocatalytic activity.


 Please wait while we load your content...
Please wait while we load your content...