Diastereoselective construction of the benzannulated spiroketal core of chaetoquadrins enabled by a regiodivergent cascade†
Abstract
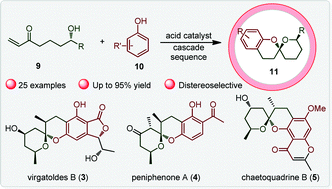
A remarkable acid-mediated methodology for the regiodivergent construction of a biologically interesting tricyclic benzannulated spiroketal skeleton with diastereomeric specificity was uncovered using a variety of readily available phenol substrates. The approach mainly refers to an intriguing hemi-ketalization/dehydration/Michael addition/ketalization cascade process, offering a viable synthetic strategy to efficiently access analogs of chaetoquadrins.


 Please wait while we load your content...
Please wait while we load your content...