Direct organocatalytic asymmetric Michael reaction of fluorine hemiaminal-type nucleophile to 4-nitro-5-styrylisoxazoles†
Abstract
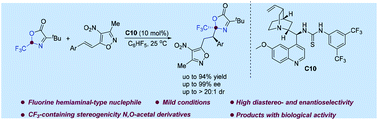
Herein, we report a chiral bifunctional thiourea catalyzed asymmetric Michael addition reaction between 2-(trifluoromethyl)oxazol-5(2H)-one as a direct C-2-position nucleophile and 4-nitro-5-styrylisoxazoles for the first time. The reaction affords the corresponding products bearing adjacent CF3-substituted hetero-quaternary and tertiary stereocenters in moderate to high yields (up to 94% yield) with good to excellent enantioselectivities (up to 99% ee) and excellent diastereoselectivities (up to >20 : 1 dr) under mild reaction conditions. Moreover, the selected compounds displayed good biological activities and further investigation is underway.


 Please wait while we load your content...
Please wait while we load your content...