Cobalt(iii)-catalyzed ketone-directed C–H vinylation using vinyl acetate†
Abstract
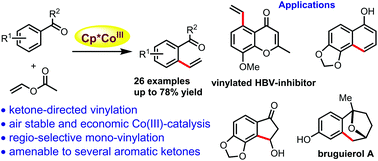
Weakly coordinating, ketone-directed C–H vinylation using vinyl acetate is reported here for a wide range of aromatic ketones such as acetophenones, diaryl ketones, chromones and biologically relevant chalcones under cost-effective and air-stable cobalt(III)-catalysis. Regioselective, mono-vinylation occurs for challenging vinyl substitution-free styrenes in moderate to good yields, and this moiety has been used to synthesize functionalized indanone, α-naphthol and an advanced intermediate for bruguierol A synthesis. An acrylate-surrogate provided the corresponding alkenylated product under these vinylation conditions. Detailed mechanistic studies are carried out to support the proposed catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...