Effective removal of uranium from aqueous solution by using novel sustainable porous Al2O3 materials derived from different precursors of aluminum†
Abstract
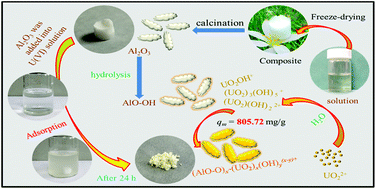
Three kinds of porous Al2O3 materials have been prepared by using different precursors of aluminum via solution-freeze-drying-calcination technology. Meanwhile, the adsorption properties of Al2O3-Cl, Al2O3-N and Al2O3-S adsorbents to U(VI) were estimated and compared. The calculated maximum adsorption amount of porous Al2O3 materials at 298 K reached 805.72 mg g−1 for U(VI) (pH = 7.0), which was much higher than that of most of the other metal oxides. Moreover, it is worth mentioning that the remarkable adsorption efficiency was above 90% even after five cycles and the adsorption efficiency was relatively high in the solution with different coexisting ions. Crucially, U(VI) was almost adsorbed completely on Al2O3-Cl at the low concentration and only about 0.006 mg L−1 remained in the solution, which was below the permissible limits of the USEPA (0.03 mg L−1) and WHO (0.015 mg L−1). Based on the above results, it could be demonstrated that the novel porous Al2O3 materials had broad application prospects for U(VI) removal.


 Please wait while we load your content...
Please wait while we load your content...