Emulsion copolymerization of vinylidene fluoride (VDF) with perfluoromethyl vinyl ether (PMVE)†
Abstract
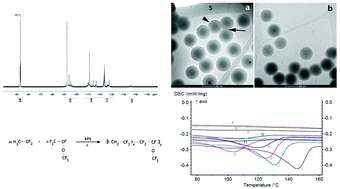
The radical emulsion copolymerization of vinylidene fluoride (VDF) with perfluoromethyl vinyl ether (PMVE), initiated by potassium persulfate in the presence or absence of a surfactant is presented. The surfactant used in this copolymerization was 3-hydroxy-2-trifluoromethyl propanoic acid (MAF-OH). The VDF mol% ranged between 60 and 90 mol%. Molar masses up to 103 000 g mol−1 with wide dispersities (Đ > 2) were produced with yields up to 74%. The composition of the resulting poly(VDF-co-PMVE) copolymers was determined by 19F NMR spectroscopy. Under certain conditions (60% VDF mol% in the autoclave inlet, MAF-OH and a low total monomer concentration), the produced latexes were stable and showed an average particle size of ca. 200 nm, as observed using a Zetasizer and a cryo-TEM. Semi-crystalline copolymers were obtained when the VDF mol% was higher than 80 mol%. These copolymers exhibit high thermostability (their degradation under air started at 400 °C). A central point 23 factorial experimental design was conducted aiming at the identification of the influence of the parameters on the reaction and the end-use properties of the polymers. Most of the studied properties were highly influenced by the VDF mol%. The highest molar mass was obtained using 90 mol% VDF in the feed, no surfactant, and a total monomer molar amount of 208 mmol, while the highest dispersity was obtained using 60 mol% VDF in the feed, no surfactant, and a total monomer molar amount of 208 mmol.


 Please wait while we load your content...
Please wait while we load your content...