Brønsted-acid-catalyzed selective Friedel–Crafts monoalkylation of isatins with indolizines in water†‡
Abstract
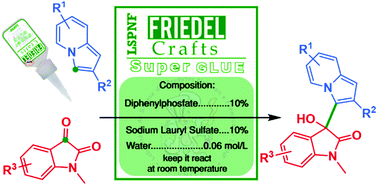
The controlled mono-addition of indolizines to isatins under very mild conditions is described. The reaction occurs in water using diphenylphosphate as the catalyst, which is dramatically accelerated by surfactant addition. 3-Hydroxy-3-indolizinyl-2-oxindole scaffolds were synthesized in up to >99% yield. Notably, in organic solvents only bis-addition products were observed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...