Asymmetric synthesis of dihydrocoumarins via catalytic sequential 1,6-addition/transesterification of α-isocyanoacetates with para-quinone methides†
Abstract
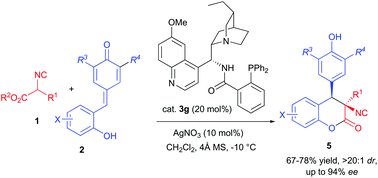
A catalytic asymmetric synthesis of 3,4-dihydrocoumarins with adjacent tertiary–quaternary stereocenters by 1,6-addition/transesterification cascade reaction of α-isocyanoacetates with ortho-hydroxyphenyl-substituted para-quinone methides (p-QMs) has been developed. Using a combination of dihydroquinidine-derived aminophosphine and silver nitrate as a binary catalytic system, moderate to good yields, excellent diastereoselectivities (>20 : 1 dr) and good to excellent enantioselectivities (up to 94% ee) were achieved for a wide range of isocyanoacetates and p-QMs.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...