Sequential multicomponent site-selective synthesis of 4-iodo and 5-iodopyrrole-3-carboxaldehydes from a common set of starting materials by tuning the conditions†
Abstract
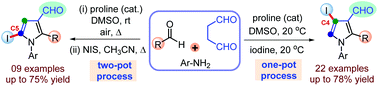
A simple and straightforward method for the synthesis of 4-iodo and 5-iodopyrrole-3-carboxaldehydes is developed from a common set of starting materials by tuning the reaction conditions. This sequential multicomponent protocol involves I2-mediated regioselective C4-iodination and aromatization of intermediate dihydropyrrole, generated through proline-catalyzed direct Mannich reaction-cyclization sequence between succinaldehyde and imines, to access 4-iodopyrroles. While aerobic oxidative aromatization of dihydropyrrole to pyrrole followed by NIS-mediated regioselective iodination furnished 5-iodopyrroles in a two-pot fashion. A series of site-selective C4/C5-iodopyrroles have been synthesized in good to high yields (up to 78%) and DFT calculations of these compounds were also performed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...