μ-Nitrido-bridged iron phthalocyanine dimer bearing eight peripheral 12-crown-4 units and its methane oxidation activity†
Abstract
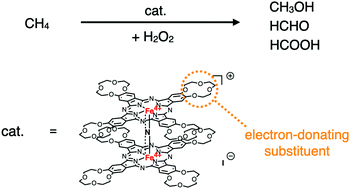
A novel μ-nitrido-bridged iron phthalocyanine dimer with eight peripheral 12-crown-4 units as an electron-donating substituent was synthesized and characterized. Examination of its methane oxidation activity in the presence of H2O2 in an acidic aqueous solution suggested that the high-valent iron oxo species generated in situ was unstable and the transiently generated decomposed species showed methane oxidation activity via Fenton-type reaction.


 Please wait while we load your content...
Please wait while we load your content...
