Acetylacetone as an oxygen activator to improve efficiency for aerobic oxidation of toluene and its derivatives by using cobalt meso-tetraphenylporphyrin
Abstract
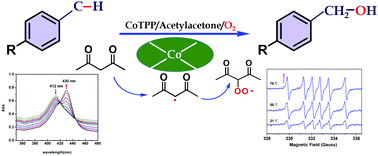
The activation of dioxygen is the major challenge in the catalytic oxygenation of hydrocarbons under mild conditions. In this study, the catalytic efficiency for the aerobic oxidation of toluene and its derivatives by using cobalt meso-tetraphenylporphyrin was enhanced by using acetylacetone as the oxygen activator. The influences of reaction conditions such as solvent, different metalloporphyrin catalysts, temperature, pressure and acetylacetone loading were studied, as well as the kinetics of the oxidation. Various toluene derivatives could also be oxidized to the corresponding products in satisfactory yields with this catalytic system. Based on the characterization of in situ electron paramagnetic resonance (EPR) and in situ UV-vis spectroscopy, the catalytic mechanism was also proposed, in which acetylacetone served as the key initiator of the free radical in activating dioxygen.


 Please wait while we load your content...
Please wait while we load your content...