Flavanthrone – a new ligand with accessible radical anion and dianion states: preparation of zwitterionic {(Cp2V)2(flavanthrone)} and {(Cp2V)2(chloranil)} complexes†
Abstract
Dye flavanthrone (Vat yellow 1) can be reduced to radical anions and dianions crystallized in the form of {Crypt(Na+)}(Flavanthrone˙−)·2C6H4Cl2 (1), (Bu4P+)2(flavanthrone2−)·0.5C6H4Cl2 (2), and (Bu4P+)2(flavanthrone2−) (3) salts, where {Crypt(Na+)} is sodium cryptate[2.2.2]. The reduction of flavanthrone is accompanied by the elongation of the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and shortening of the C–C(O) bonds. The reduction is manifested in optical spectra by an essential (more than 200 nm) red shift of the intense band of pristine flavanthrone at 440 nm. A new intense band appears for the flavanthrone˙− radical anion at 960 nm in the NIR range but this band decreases in intensity for dianion salts 2 and 3. Salt 1 contains flavanthrone˙− chains of three types in which they are bonded by C
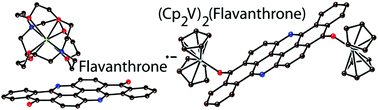
O bonds and shortening of the C–C(O) bonds. The reduction is manifested in optical spectra by an essential (more than 200 nm) red shift of the intense band of pristine flavanthrone at 440 nm. A new intense band appears for the flavanthrone˙− radical anion at 960 nm in the NIR range but this band decreases in intensity for dianion salts 2 and 3. Salt 1 contains flavanthrone˙− chains of three types in which they are bonded by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H hydrogen bonds of 2.26–2.53 Å length. Although direct π–π interactions between flavanthrone˙− chains (S = 1/2) are absent, hydrogen bonds between them provide weak antiferromagnetic coupling of spins in the flavanthrone˙− layers with a Weiss temperature of −5 K. Flavanthrone2− dianions are diamagnetic and EPR silent. The interaction of flavanthrone with two equivalents of vanadocene yields a zwitterionic {(Cp2V+)2(flavanthrone2−)}·2C6H4Cl2 (4) complex bonded by short V–O(flavanthrone) bonds. These molecules are packed in uniform stacks with effective π–π interactions between flavanthrone2− units (having an interplanar distance of 3.38 Å). Paramagnetic VIII centers with an S = 1 spin state are separated in 4 by diamagnetic flavanthrone2− dianions and show weak magnetic coupling with a Weiss temperature of −5 K. Zwitterionic {(Cp2V+)2(QCl42−)} complex (5) with a smaller diamagnetic chloranil2− spacer shows slightly stronger magnetic coupling with a Weiss temperature of −7 K. Modeling of the magnetic behavior of 4 and 5 using the appropriate van Vleck equation indicates stronger magnetic coupling between the VIII centers in 5 (J = −3.3 cm−1) in comparison with 4 (J = −2.0 cm−1).
O⋯H hydrogen bonds of 2.26–2.53 Å length. Although direct π–π interactions between flavanthrone˙− chains (S = 1/2) are absent, hydrogen bonds between them provide weak antiferromagnetic coupling of spins in the flavanthrone˙− layers with a Weiss temperature of −5 K. Flavanthrone2− dianions are diamagnetic and EPR silent. The interaction of flavanthrone with two equivalents of vanadocene yields a zwitterionic {(Cp2V+)2(flavanthrone2−)}·2C6H4Cl2 (4) complex bonded by short V–O(flavanthrone) bonds. These molecules are packed in uniform stacks with effective π–π interactions between flavanthrone2− units (having an interplanar distance of 3.38 Å). Paramagnetic VIII centers with an S = 1 spin state are separated in 4 by diamagnetic flavanthrone2− dianions and show weak magnetic coupling with a Weiss temperature of −5 K. Zwitterionic {(Cp2V+)2(QCl42−)} complex (5) with a smaller diamagnetic chloranil2− spacer shows slightly stronger magnetic coupling with a Weiss temperature of −7 K. Modeling of the magnetic behavior of 4 and 5 using the appropriate van Vleck equation indicates stronger magnetic coupling between the VIII centers in 5 (J = −3.3 cm−1) in comparison with 4 (J = −2.0 cm−1).


 Please wait while we load your content...
Please wait while we load your content...
