Chemo-, regio-, and stereoselectivity in 1,3-dipolar cycloaddition of piperine with nitrones. A cycloadditive route to aminoalcohols†
Abstract
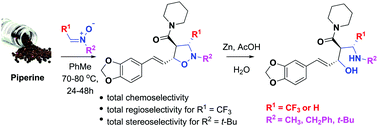
Prochiral N-alkyltrifluoromethylmethylene nitrones reacted smoothly with piperine, an α,β,γ,δ-unsaturated natural amide, to afford novel corresponding cycloadducts in moderate yields. This non-catalysed 1,3-dipolar cycloaddition was an entirely chemo- and regioselective reaction, and its stereoselectivity was strongly dependent on both the steric hindrance of the nitrone and the effects of the electron-withdrawing CF3 group. The major products 6a–6c were found to have the C3/C4 trans relative configuration of the protons in the isoxazolidinyl ring. In contrast, the 1,3-dipolar cycloaddition of unsubstituted N-methylmethylene nitrone to piperine led to two regioisomeric cycloadducts 7 and 8. The major cycloadducts underwent N–O cleavage todiastereoselectively pure 1,3-aminoalcohols 10a, 10c and 10d. The structure of the products was established by spectroscopic techniques, and the stereochemical aspects were discussed based on 1D/2D NMR relationships.


 Please wait while we load your content...
Please wait while we load your content...