Lewis acid induced spectral changes of sterically hindered and unhindered meso-tetra(aryl)porphyrins: fluorescence emission spectra†
Abstract
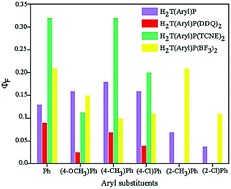
The formation of molecular complexes between porphyrins and Lewis acids influences both the absorption and emission spectra of these compounds. In the present study, the molecular complexes of a series of sterically hindered and unhindered meso-tetra(aryl)porphyrins (aryl = phenyl, 4-methoxyphenyl, 4-methylphenyl, 4-chlorophenyl, 2-methylphenyl and 2-chlorophenyl) with BF3, tetracyanoethylene (TCNE) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) were used to investigate the effects of molecular complexation on the fluorescence emission properties of the porphyrins. Different parameters including the wavelength and intensity of the emission bands, fluorescence quantum yield (ΦF), lifetime of the S1 excited state (τ) as well as the radiative and non-radiative decay rate constants (kr and knr) were determined and compared. In spite of the close resemblance between the absorption spectra of these molecular complexes, large differences were observed between their emission spectra. In other words, fluorescence spectroscopy is much more sensitive to the nature of Lewis acid than UV-vis absorption spectroscopy. Also, it was found that the presence of bulky substituents at the meso positions prevents the formation of stable molecular complexes with TCNE and DDQ.


 Please wait while we load your content...
Please wait while we load your content...