Activation of small molecules by cyclic alkyl amino silylenes (CAASis) and germylenes (CAAGes): a theoretical study†
Abstract
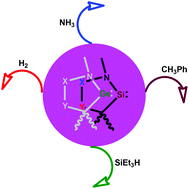
Quantum chemical calculations have been carried out on a series of skeletally modified cyclic alkyl amino silylenes (CAASis) and germylenes (CAAGes) to understand their ligand properties and reactivity towards the activation of a variety of small molecules. The installation of boron or silicon atoms into the ring framework of these silylenes/germylenes led to a dramatic increase in their σ-basicity while the incorporation of ylidic moieties resulted in a sharp reduction of their π-acidity although it did help in increasing the electron donation ability. The calculated values of energy barriers for the activation of H–H, N–H, C–H and Si–H bonds by many of the cyclic silylenes considered here are found to be comparable to those for experimentally evaluated systems, indicating the potential of these computationally designed molecules in small molecule activation and calling for synthetic efforts towards their isolation. Furthermore, activations employing CAAGes are found to be more demanding than those with CAASis which may be attributed to the significantly lower Lewis basicity of the former than the latter.


 Please wait while we load your content...
Please wait while we load your content...