A new synthesis of porphyrins via a putative trans-manganese(iv)-dihydroxide intermediate†
Abstract
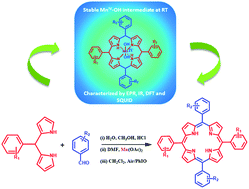
A new method for the synthesis of meso-substituted porphyrins was developed. In this two-step methodology, the first step involves the condensation of pyrroles/dipyrromethanes with aldehydes in a water–methanol mixture under acidic conditions. The second step involves manganese induced cyclization followed by oxidation via PhIO/O2. This methodology has been useful for the synthesis of a wide range of trans-A2B2 porphyrins and also symmetric porphyrins in moderate to good yields. A detailed investigation of the manganese induced cyclization reaction has allowed us to characterize a Mn–porphyrinogen complex. A series of analytical and spectroscopic techniques and DFT calculations have led us to the conclusion that the putative intermediate species are trans-manganese(IV)-dihydroxide complexes. EPR and magnetic susceptibility measurements helped us to assign the oxidation state of the manganese complexes in their native state. The assumption of trans-manganese(IV)-dihydroxide as the true intermediate for this porphyrin synthesis has been authenticated via in situ UV-Vis experiments. This new methodology is certainly different from other previously reported methodologies in many aspects and most importantly these reactions can be easily performed on a gram scale for the synthesis of porphyrins.


 Please wait while we load your content...
Please wait while we load your content...