Single-molecule level dynamic observation of disassembly of the apo-ferritin cage in solution†
Abstract
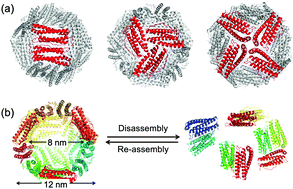
The ferritin cage iron-storage protein assembly has been widely used as a template for preparing nanomaterials. This assembly has a unique pH-induced disassembly/reassembly mechanism that provides a means for encapsulating molecules such as nanoparticles and small enzymes for catalytic and biomaterial applications. Although several researchers have investigated the disassembly process of ferritin, the dynamics involved in the initiation of the process and its intermediate states have not been elucidated due to a lack of suitable methodology to track the process in real-time. We describe the use of high-speed atomic force microscopy (HS-AFM) to image the dynamic event in real-time with single-molecule level resolution. The HS-AFM movies produced in the present work enable direct visualization of the movements of single ferritin cages in solution and formation of a hole prior to disassembly into subunit fragments. Additional support for these observations was confirmed at the atomic level by the results of all-atom molecular dynamics (MD) simulations, which revealed that the initiation process includes the opening of 3-fold symmetric channels. Our findings provide an essential contribution to a fundamental understanding of the dynamics of protein assembly and disassembly, as well as efforts to redesign the apo-ferritin cage for extended applications.


 Please wait while we load your content...
Please wait while we load your content...