Electronic structure, ion diffusion and cation doping in the Na4VO(PO4)2 compound as a cathode material for Na-ion batteries†
Abstract
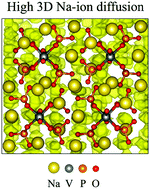
Sodium-ion batteries are considered one of the most promising alternatives to lithium-ion batteries owing to the low cost and wide abundance of sodium. Phosphate compounds are promising materials for sodium-ion batteries because of their high structural stability, energy densities and capacities. Vanadium phosphates have shown high energy densities, but their sodium-ion diffusion and cation doping properties are not fully rationalized. In this work, we combine density functional theory calculations and molecular dynamics simulations to study the electronic structure, ion diffusion and cation doping properties of the Na4VO(PO4)2 compound. The calculated Na-ion activation energy of this compound is 0.49 eV, which is typical for Na-based cathode materials, and the simulations predict a Na-ion diffusion coefficient of 5.1 × 10−11 cm2 s−1. The cell voltage trends show a voltage of 3.3 V vs. Na/Na+. Partial substitution of vanadium atoms by other metals (Al3+, Co2+, Fe3+, Mn4+, Ni2+ or Ti4+) increases the cell voltage up to 1.1 V vs. Na/Na+. These new insights will help us to understand the ion transport and electrochemical behaviour of potential phosphate cathode materials for sodium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...