Synthesis of functionalized cyclopropylboronic esters based on a 1,2-metallate rearrangement of cyclopropenylboronate†
Abstract
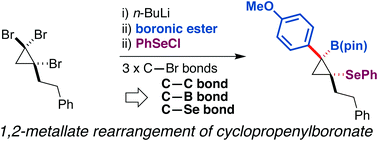
A procedure converting tribromocyclopropane to densely functionalized β-selenocyclopropylboronic ester using the 1,2-metallate rearrangement of a boron ate-complex has been developed. Treatment of an in situ-generated cyclopropenylboronic ester ate-complex with phenylselenenyl chloride triggered stereospecific rearrangement to produce functionalized cyclopropanes. DFT calculations for 1,2-metallate rearrangement suggested that the reaction proceeds through a seleniranium intermediate.


 Please wait while we load your content...
Please wait while we load your content...