Alteration of water absorption in the THz region traces the onset of fibrillation in proteins†
Abstract
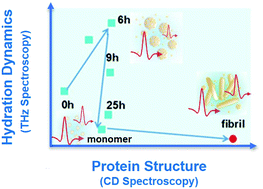
Using terahertz spectroscopy, we established the alteration of the collective hydration of water during the fibrillation process (native → intermediate → fibril) of a model protein bovine serum albumin. This label-free study concludes that water dynamics change systematically with protein conformational changes as it experiences a hydrophobic environment during the initial protein unfolding process, followed by the release of bound water during oligomerization and finally the hydrophobic interior of the fibril.


 Please wait while we load your content...
Please wait while we load your content...