Selective functionalization at N2-position of guanine in oligonucleotides via reductive amination†
Abstract
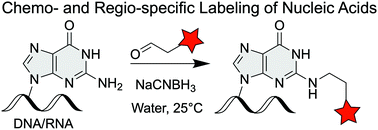
Chemo- and site-specific modifications in oligonucleotides have wide applicability as mechanistic probes in chemical biology. However, methods that label specific sites in nucleic acids are scarce, especially for labeling DNA/RNA from biological or enzymatic sources rather than synthetic ones. Here we have employed a classical reaction, reductive amination, to selectively functionalize the N2-amine of guanosine and 2′-deoxyguanosine monophosphate (GMP/dGMP). This method specifically modifies guanine in DNA and RNA oligonucleotides, while leaving the other nucleobases unaffected. Using this approach, we have successfully incorporated a reactive handle chemoselectively into nucleic acids for further functionalization and downstream applications.


 Please wait while we load your content...
Please wait while we load your content...