Oxidation of 8-thioguanosine gives redox-responsive hydrogels and reveals intermediates in a desulfurization pathway†
Abstract
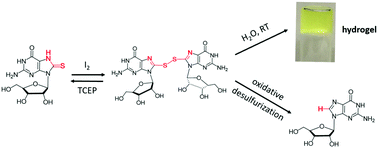
A disulfide made by oxidation of 8-thioguanosine is a supergelator. The hydrogels are redox-responsive, as they disassemble upon either reduction or oxidation of the S–S bond. We also identified this disulfide, and 2 other compounds, as intermediates in oxidative desulfurization of 8-thioG to guanosine.


 Please wait while we load your content...
Please wait while we load your content...
