Iodine promoted cascade cycloisomerization of 1-en-6,11-diynes†
Abstract
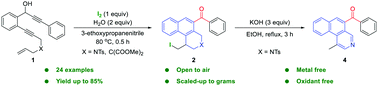
An iodine promoted cascade cycloisomerization of 1-en-6,11-diynes is presented for the easy preparation of tetrahydrobenzo[f]isoquinolines. This developed reaction system is identified as having good functional-group applicability and can be scaled up to gram quantities. In this transformation, two new cyclic frameworks and one carbonyl group are formed with four new bonds constructed. Additionally, the resulting iodo-substituted compounds could be further derived through simple elimination reactions.


 Please wait while we load your content...
Please wait while we load your content...