A rapid, multiplexed kinase activity assay using 8-plex iTRAQ labeling, SPE, and MALDI-TOF/TOF MS†
Abstract
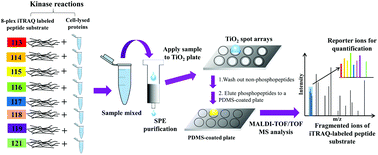
Synthesized peptide substrates have been used for in vitro phosphorylation using purified kinases or cell lysates. For screening assays, a direct readout and comparison among different experimental conditions without using an internal standard would be preferred. In this study, we developed a rapid, quantitative measurement method of multikinase activity based on MALDI-TOF/TOF MS. We combined 8-plex iTRAQ-labeled peptide substrates, solid phase extraction (SPE), and a phosphorylated peptide purification plate to rapidly determine multikinase activity in cell lysates. To enable our platform to be applicable in insulin stimulation and cancer drug inhibition, a list of peptide substrates was designed. By labeling peptide substrates with 8-plex iTRAQ reagents, protein kinase activity in 8 samples could be directly compared using the mass tags on their fragmented ion spectra. The protein amount and incubation time for multikinase activity assays were optimized, and the effect of insulin stimulation and an inhibitory drug on the cellular protein kinase activity was evaluated.


 Please wait while we load your content...
Please wait while we load your content...