A p-π* conjugated triarylborane as an alcohol-processable n-type semiconductor for organic optoelectronic devices†
Abstract
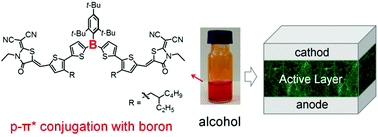
We report a p-π* conjugated organic molecule based on triarylborane as n-type organic semiconductor with unique alcohol solubility. Its favorable alcohol solubility even in the absence of polar side chains is mainly due to the large dipole moment and enhanced flexibility of the conjugated backbone once the boron atom is embedded. The p-π* conjugation directly affects the electronic structure as the LUMO is fully delocalized, including the boron atom, whereas the HOMO has the boron atom residing on a node. As a result, the molecule exhibits low-lying LUMO/HOMO energy levels of −3.61 eV/−5.73 eV paired with a good electron mobility of 1.37 × 10−5 cm2 V−1 s−1. We further demonstrate its application as an electron acceptor in alcohol-processed organic solar cells (OSCs). To our best knowledge, this p-π* conjugated molecule is the first alcohol-processable non-fullerene electron acceptor, a feature that is in strong demand for environmentally friendly processing of OSCs.


 Please wait while we load your content...
Please wait while we load your content...