Understanding on the structural and electrochemical performance of orthorhombic sodium manganese oxides†
Abstract
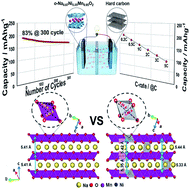
We investigate the orthorhombic Na0.67[NixMn1−x]O2 (x = 0 and 0.05) cathode materials that provide high capacity for prolonged cycles. X-ray absorption studies revealed that the redox activity of the Mn3+/4+ and Ni2+/3+ pairs is effective in suppressing the Jahn–Teller effect of Mn3+ ions because of the network with Ni2+ ions. This effect influenced the smooth voltage variations in the voltage profile for Na0.67[Ni0.05Mn0.95]O2, whereas several complicated voltage plateaus associated with the first-order phase transition were noticed in Na0.67MnO2. Operando synchrotron X-ray diffraction and transmission microscopy studies confirmed the simplicity of the phase transition for Na0.67[Ni0.05Mn0.95]O2 due to suppression of the Jahn–Teller effect of Mn3+ in the oxide lattice. These findings, along with the capacity retention during prolonged cycling and the acceptable thermal properties, make high-capacity sodium-ion batteries feasible, inexpensive, and safe for energy storage application.


 Please wait while we load your content...
Please wait while we load your content...