Correlation between Ru–O hybridization and the oxygen evolution reaction in ruthenate epitaxial thin films†
Abstract
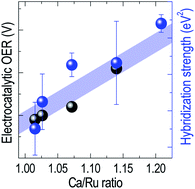
Hybridization of orbitals determines the electronic structure of transition metal oxides and strongly influences the formation and breaking of chemical bonds necessary for electrocatalytic activity. However, the exact relation between p–d hybridization and electrocatalytic activity is yet to be elucidated due to the lack of selective controllability over hybridization. Here, the electronic structure and electrocatalytic behavior of epitaxial CaRuO3 thin films are studied, to clarify the correlation between hybridization and the oxygen evolution reaction. A decreased hybridization strength between Ru 4d and O 2p states leads to a significant enhancement in the oxygen evolution reaction. A strong hybridization within the CaRuO3 thin film is found to hinder the formation of chemical bonds between the transition metal and adsorbate molecules, thereby decreasing the electrocatalytic efficiency. Our study provides a rigorous correlation between the electrocatalytic activity and electronic structure, taking advantage of the selective hybridization strength control offered by the CaRuO3 system.


 Please wait while we load your content...
Please wait while we load your content...