A highly enantioselective synthetic method towards the α2c-adrenoceptor antagonist ORM-10921†
Abstract
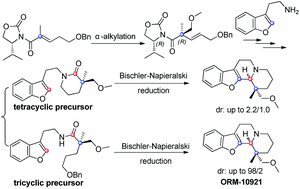
The preparation of ORM-10921, a selective α2C-adrenoceptor antagonist with promising anti-psychotic properties, was successfully achieved using asymmetric α-alkylation of α,β-unsaturated imide and Bischler–Napieralski cyclization/asymmetric reduction as the key steps. When the tetracyclic iminium 11S was used in the reduction, the diastereo-selectivity was poor, from which four stereoisomers of ORM-10921 were obtained, respectively. However, the diastereoselectivity could be significantly improved (with dr up to >97 : 3) when the tricyclic imine substrate 19S was applied in this reduction, suggesting an additional chelation from the side-chain methoxy group in the transition state. According to this protocol, ORM-10921 was accomplished in a highly enantioselective manner. In addition, two analogs 26Aa and 26Ba were prepared using this novel method, and the absolute configurations were unambiguously assigned by single crystal X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...