Helicity induction and memory effect in poly(biphenylylacetylene)s bearing various functional groups and their use as switchable chiral stationary phases for HPLC†
Abstract
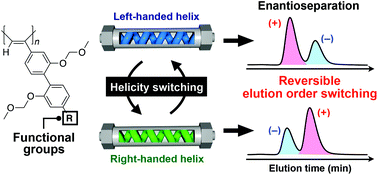
A series of poly(biphenylylacetylene)s (PBPAs) bearing various functional groups at the 4′-position of the biphenyl pendants were synthesized. The effects of the pendant functional groups on their macromolecular helicity induction and subsequent memory behaviors as well as their chiral recognition abilities as chiral stationary phases (CSPs) for high-performance liquid chromatography (HPLC) were investigated. Both the macromolecular helicity and the pendant axial chirality of the PBPAs bearing ester, acyloxy or carbamate groups induced by noncovalent interactions with an optically active alcohol were more stably memorized compared to those induced in the PBPAs bearing ether pendant groups when the optically active alcohol was completely removed. The PBPAs with a macromolecular helicity memory showed different chiral recognition abilities toward various racemates depending on the difference in the achiral pendant functional groups when used as CSPs for HPLC under normal phase conditions. The PBPA bearing carbamate pendants exhibited an excellent chiral recognition ability and resolved many racemates including axially chiral compounds and metal acetylacetonate complexes. Since the obtained PBPAs are soluble in the chiral alcohol, it was almost impossible to switch their enantioselectivities by treatment of the coated-type CSPs with a solution of the chiral alcohol in the column. By immobilizing the PBPA chains onto silica gel, the reversible control of the macromolecular helicity in the column could be achieved through alternate column treatment with a solution containing the (R)- or (S)-enantiomer of the chiral alcohol, thereby enabling the repeated switching of the enantiomer elution order.


 Please wait while we load your content...
Please wait while we load your content...