Blue fluorescence from N,O-coordinated BF2 complexes having aromatic chromophores in solution and the solid state†
Abstract
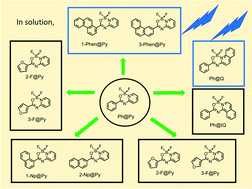
We prepared amide-heterocycle (HC) compounds having various aromatic π-electron systems (Ar), such as phenyl, naphthyl, furyl, thienyl and phenanthryl moieties, and converted them as ligands to difluoroboronated complexes, Ar@HCs. Blue fluorescence from Ar@HCs was observed in solution and the solid state, and the fluorescence quantum yields (Φf) and lifetimes (τf) were determined. The Φf values in CHCl3 were as small as 0.1 except for the phenanthrene derivatives (0.4–0.6). Observation of the triplet–triplet absorption upon laser flash photolysis of Ar@HCs in solution indicated that the fluorescence process competes with intersystem crossing to the triplet state. Blue fluorescence in the solid state was observed with the Φf values of 0.3–0.7. Based on the crystallographic data, the relationship between the crystal structures and emission features of Ar@HCs in the solid state is discussed.


 Please wait while we load your content...
Please wait while we load your content...